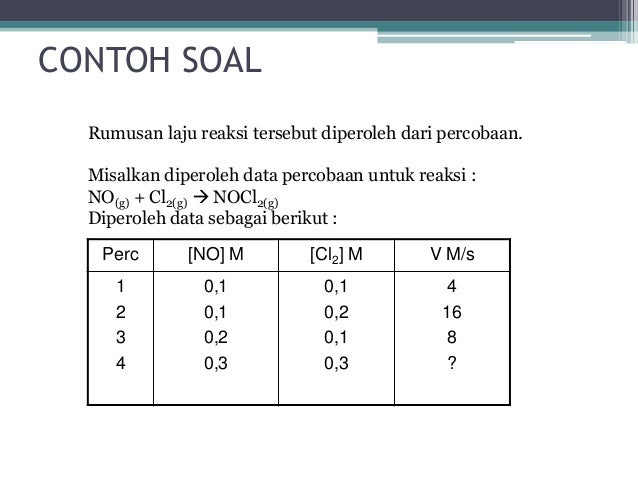
Contoh Soal Laju Reaksi
Konstanta laju reaksi (k) adalah suatu tetapan yang harganya bergantung pada jenis perekasi, suhu dan katalis. Konstanta laju reaksi adalah perbandingan antara laju reaksi dengan konsentrasi reaktan. Setiap reaksi mempunyai harga k tertentu pada suhu tertentu. Harga k ini akan berubah jika suhunya berubah. Suatu reaksi berlangsung dua kali lipat lebih cepat setiap suhunya dinaikkan 10˚C. Jika laju reaksi saat suhu 40˚C adalah M S-1, laju reaksi saat suhu dinaikkan menjadi 120˚C adalah a. Pembahasan: selanjutnya → Latihan Soal 16-25 Laju Reaksi Serta Pembahasan Soal. V = laju reaksi k = ketetapan laju reaksi m = orde raksi terhadap A n = order reaksi terhadap B m + n = orde reaksi Berdasarkan rumus di atas, maka persamaan laju reaksi untuk reaksi 2A(g) + B(g) → 2AB(g) dapat ditulis sebagai: ⇒ v = kA m B n Nah, karena m dan n belum diketahui, maka kita harus mencari nilai kedua orde tersebut.
All video source collected from YouTube.2. We are using wp-automatic plug-in which periodically search and post YouTube video automatically with keyword “soal cpns” and “contoh soal cpns”.3. All the video content owned by YouTube uploader/video creator. We don’t owned or create all the video that posted here.4. We are not take any responsibility for the video content. All YouTube video claim should go to video owner/YouTube uploader/video creator)5. We only search and post video content from youtube (point 1 and 2).
We don’t own any of the video that posted here (point 3) so all claim and responsibility should go to video owner/YouTube uploader/video creator (point 4).
Modul persamaan laju reaksi.1.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 201001/30/15 1Dr. Masykuri, M.Si.Chemical Education Study ProgramTeacher Training and Educational StudiesSebelas Maret University (UNS)Website: mmasykuri@yahoo.comSolo, Sep 2010Kimia Fisika IV.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Rate of ReactionConsider a chemical reaction having the overallstoichiometry:aA + bB → cC + dDThe Rate of Reaction is defined asExperimentally we find thatk = rate coefficientCi = Concentration of Reactant “i” or iγi = Order of reaction with respect to reactant “i”.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Macam MetodeMetode Penentuan Tetapan Laju:Metode relaksasi, metode analisis guegenheim.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Metode Diferensial. Data dikumpulkan sebagai laju perubahankonsentrasi/waktu terhadap konsentrasi reaktan.
Contoh dasar persamaan bagi reaksi dengan 2 reaktan:aA + bB → produkqpBAkdtAdr =−=lnlnlnlnln BqApkdtAdr =−=. Dibuat kurva ln r terhadap ln A atau ln B.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Metode Diferensiallnlnlnlnln BqApkdtAdr =−=. Kurva ln r terhadap ln Aln'lnln Apkr +=Orde reaksi terhadap A = p, diperoleh dari slope = tg αTetapan laju k, diperoleh dari intersep grafikOrde reaksi terhadap A = p, diperoleh dari slope = tg αTetapan laju k, diperoleh dari intersep grafikSlope = tg α= plnln'ln BqkkIntersep +.Teacher Training and Educational StudiesSebelas Maret UniversityM.
It certainly sounds like a city-building game, except done from the ground level – and is another example of of some of these Skyrim modders. Skyrim small house mod. It starts you off as a peasant with a single shed, and from there you have to build up until you have your own castle, and town filled with NPCs that you rule over as noble lord.You can pick up the LCBuild Your Noble House mod. After gathering materials to build all these things you can get NPCs to work for you, or rent out buildings, and they’ll produce money or resources. You don’t even have to do everything yourself either.

MasykuriPhysical Chemistry IVSep, 2010Metode DiferensialData konsentrasi zat A pada reaksi,yang dikatalisis suatu katalis C sebagai fungsi waktu diketahui sbb:A → produkContoh:t (menit) A (M) t (menit) A (M)0 0,800 8 0,3822 0,647 10 0,3294 0,534 12 0,2866 0,448 14 0,252a. Tentukan orde reaksiterhadap A sampai kelipatan½ terdekatb. Tentukan harga tetapan lajureaksi k dengan satuannyayang tepat.Teacher Training and Educational StudiesSebelas Maret UniversityM.
MasykuriPhysical Chemistry IVSep, 2010Metode Integral. Bersifat trial and error (empiris), yaitu denganmemasukkan data pada persamaan laju bentuk integral. Jika harga k relatif tetap (konstan), maka orde reaksinyasesuai. Penentuan empiris orde reaksi terhadap reaktan:– Orde nol– Orde pertama– Orde kedua tipe I– Orde kedua tipe II– Prde ketiga.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Metode Integral. The rate of the reaction is independent of theconcentration of the reacting substanceZero Order ReactionsA → products∫∫ −=−−dtkAdkdtAdkdtAdAA 0At - A0 = - ktThe reaction half-time t1/2 is the timerequired for the concentration todecrease to one-half of its initial value.Teacher Training and Educational StudiesSebelas Maret UniversityM.
Tetapan Laju Reaksi
MasykuriPhysical Chemistry IVSep, 2010Metode IntegralZero Order ReactionsGrafik A versus t,Persamaan grafik,Slope = - kIntersep = A0Waktu paruh,.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Metode IntegralFirst Order ReactionsThe rate of the reaction is proportional to the first power of theconcentration of the reacting substance (order = 1)A → products∫∫ −=−−dtkAAdkdtAAdAkdtAdAA 0Examples:.
decay of radioactive nuclei fluorescence. decay of electronically excited molecules. isomerization of cyclobutene to butadiene. milk turns sour when left out overnight.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Metode IntegralFirst Order ReactionsGrafik ln A versus t,Persamaan grafik,Buktikan bahwa,.Teacher Training and Educational StudiesSebelas Maret UniversityM.
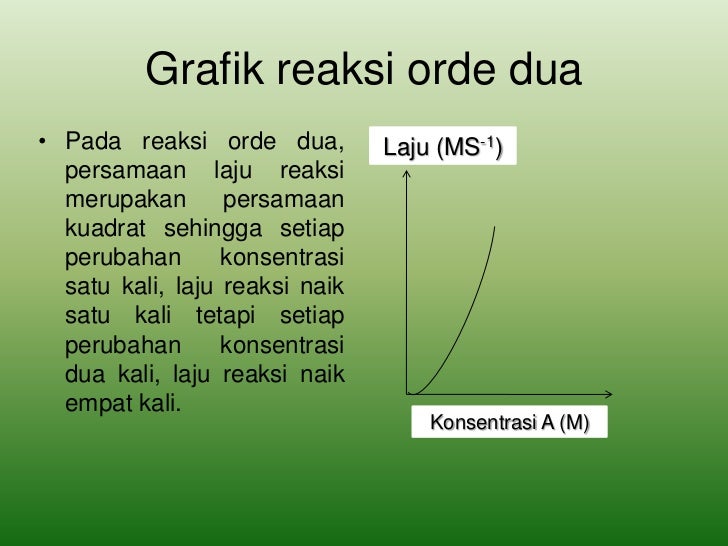
Contoh Soal Laju Reaksi Orde 2
MasykuriPhysical Chemistry IVSep, 2010Metode IntegralSecond Order ReactionsThe rate of the reaction is proportional to the second power ofthe concentration of one reactant or to the first power of theconcentrations of two reactants (order = 2)a) Second order in one componenta) Second order in one componentA → productsb) First order in each of two componentsb) First order in each of two componentsA +B → products.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010Metode IntegralSecond Order Reactionsa) Second order in one componenta) Second order in one componentA → products.Teacher Training and Educational StudiesSebelas Maret UniversityM. MasykuriPhysical Chemistry IVSep, 2010b) First order in each of two componentsb) First order in each of two componentsMetode IntegralSecond Order ReactionsA +B → productsSpecial cases:i) Ao = Boii) Bo.